行业动态
Contact Us
北京博力康宁环保技术咨询有限公司
传真:010-8767-6595
电话:010-8767-1126(国内)
电话:+86-10-8767-1126(国外)
E-MAIL:lenaliu@bjboardingcard.com
地址:北京市朝阳区瑞安大厦1123室
传真:010-8767-6595
电话:010-8767-1126(国内)
电话:+86-10-8767-1126(国外)
E-MAIL:lenaliu@bjboardingcard.com
地址:北京市朝阳区瑞安大厦1123室
New Statistics on Food Related/Contact Material Cases in China
2019-06-11 10:18:12
点击数:
Dr. Zhu Lei from China Food Safety Risk Assessment Center (CFSA) recently summarized the progress on new food-related/ contact materials (FCM) petition in China in recent years at the first “National Forum on Food Safety and Nutrition, and on Food Safety Risk Prevention and Control" held in Jinan, Shandong province at the end of May.
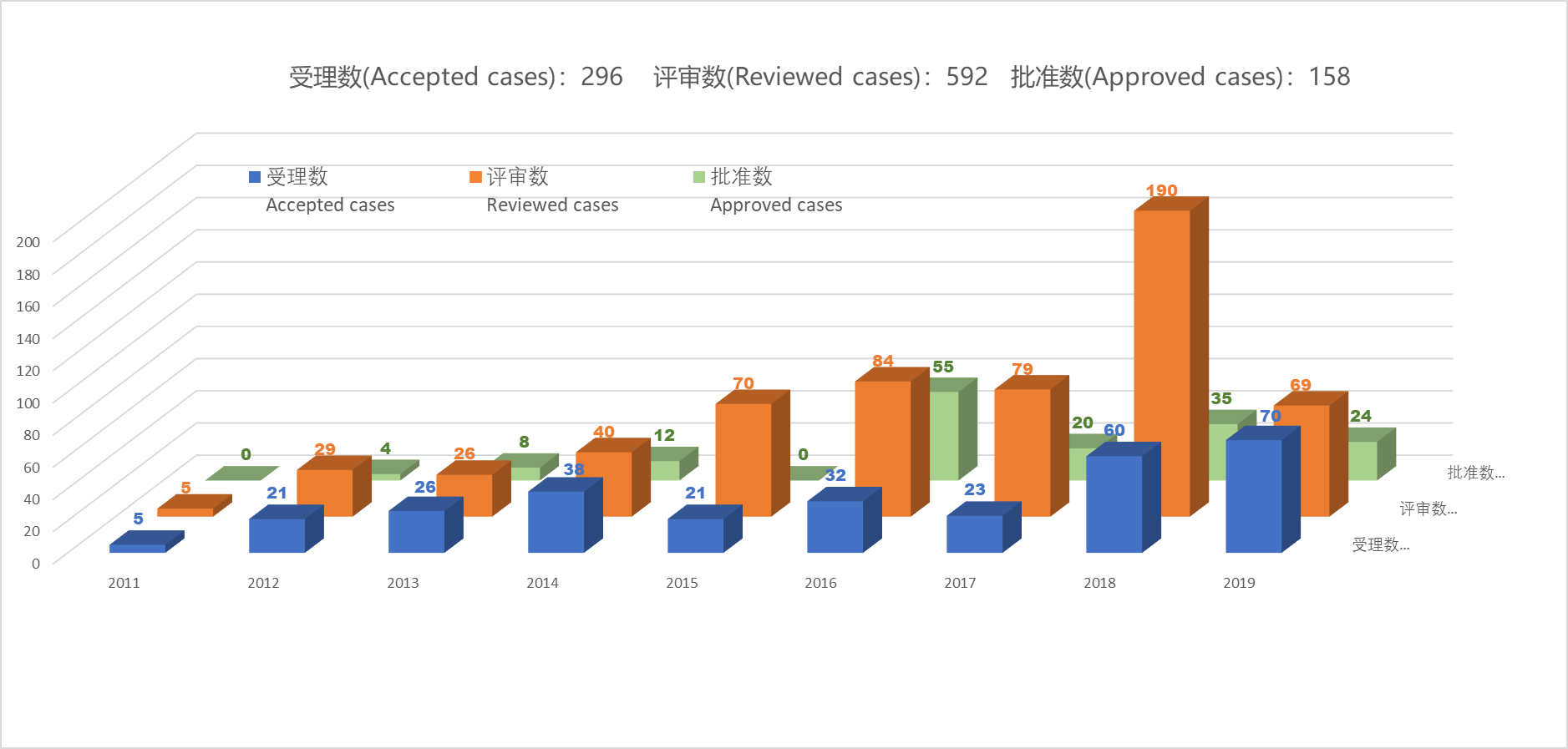
According to the statistical data, since officially launched in 2011 the number of FCM petitions has been increasing year by year. Although there were some setbacks from the second half of 2014 to the first half of 2016, which made the petition work somewhat stalled, the normal administration pace was resumed at the end of 2016. After a series of new national standards (GB) came into effect in 2017, the number of FCM petitions in 2018 increased significantly, approaching to 60 substances altogether. And the number grows even faster recently, only in the first five months of 2019 the cases submitted has exceeded that of the whole year of 2018, reaching 70 substances.
In addition, on May 20th this year, another thirty-six national food safety standards were released for public opinions, including the draft on food contact adhesives etc., which might trigger a new wave of petitions.
The increase in the number of applications also poses some challenges for authorities and review experts. The figure in the chart shows that there were 190 cases reviewed in 2018. This means, on average, experts are required to evaluate about 30 submissions per review meeting, including first submitted dossiers and supplementary materials for previous cases. Therefore, Dr. Zhu Lei also pointed out that the whole operation mode has to be further optimized and technical guidelines to be improved.
So far, 296 substances have been accepted by authority and 158 have been approved, with an approval rate of 53%. Combined with the total number of reviews (592), it can be deduced that 2-3 rounds of review are expected before a case can be final approved. When planning the time to market, applicants shall fully consider the time lost caused by uncertain factors and reserve sufficient petition dossier preparation time.

According to the statistical data, since officially launched in 2011 the number of FCM petitions has been increasing year by year. Although there were some setbacks from the second half of 2014 to the first half of 2016, which made the petition work somewhat stalled, the normal administration pace was resumed at the end of 2016. After a series of new national standards (GB) came into effect in 2017, the number of FCM petitions in 2018 increased significantly, approaching to 60 substances altogether. And the number grows even faster recently, only in the first five months of 2019 the cases submitted has exceeded that of the whole year of 2018, reaching 70 substances.
In addition, on May 20th this year, another thirty-six national food safety standards were released for public opinions, including the draft on food contact adhesives etc., which might trigger a new wave of petitions.
The increase in the number of applications also poses some challenges for authorities and review experts. The figure in the chart shows that there were 190 cases reviewed in 2018. This means, on average, experts are required to evaluate about 30 submissions per review meeting, including first submitted dossiers and supplementary materials for previous cases. Therefore, Dr. Zhu Lei also pointed out that the whole operation mode has to be further optimized and technical guidelines to be improved.
So far, 296 substances have been accepted by authority and 158 have been approved, with an approval rate of 53%. Combined with the total number of reviews (592), it can be deduced that 2-3 rounds of review are expected before a case can be final approved. When planning the time to market, applicants shall fully consider the time lost caused by uncertain factors and reserve sufficient petition dossier preparation time.


